In its strictest sense, petroleum includes only crude oil, but in common usage it includes both crude oil and natural gas. Both crude oil and natural gas are predominantly a mixture of hydrocarbons. Under surface pressure and temperature conditions, the lighter hydrocarbons methane, ethane, propane and butane occur as gases, while the heavier ones from pentane and up are in the form of liquids or solids. However, in the underground oil reservoir the proportion which is gas or liquid varies depending on the subsurface conditions, and on the phase diagram of the petroleum mixture.
An oil well produces predominantly crude oil, with some natural gas dissolved in it. Because the pressure is lower at the surface than underground, some of the gas will come out of solution and be recovered (or burned) as associated gas or solution gas. A gas well produces predominately natural gas. However, because the underground temperature and pressure are higher than at the surface, the gas may contain heavier hydrocarbons such as pentane, hexane, and heptane in the gaseous state. Under surface conditions these will condense out of the gas and form natural gas condensate, often shortened to condensate. Condensate resembles gasoline in appearance and is similar in composition to some volatile light crude oils.
The proportion of light hydrocarbons in the petroleum mixture is highly variable between different oil fields and ranges from as much as 97% by weight in the lighter oils to as little as 50% in the heavier oils and bitumens.
Crude oil varies greatly in
appearance depending on its composition. It is usually black or dark brown
(although it may be yellowish or even greenish). In the reservoir it is
usually found in association with
natural gas, which
being lighter forms a gas cap over the petroleum, and
saline water which,
being heavier than most forms of crude oil, generally sinks beneath it.
Crude oil may also be found in semi-solid form mixed with sand and water, as
in the
Athabasca oil sands in
Canada, where it is
usually referred to as crude
bitumen. In Canada,
bitumen is considered a sticky, tar-like form of crude oil which is so thick
and heavy that it must be heated or diluted before it will flow.
Venezuela also has large amounts of oil in the
Orinoco oil sands,
although the hydrocarbons trapped in them are more fluid than in Canada and
are usually called
extra vheay oil. These
oil sands resources are called
unconventional oil to
distinguish them from oil which can be extracted using traditional oil well
methods. Between them, Canada and
Venezuela contain an
estimated 3.6 trillion barrels (570×10^9 m3)
of bitumen and extra-heavy oil, about twice the volume of the world's
reserves of conventional oil.
Petroleum is used mostly, by volume, for producing fuel oil and gasoline, both
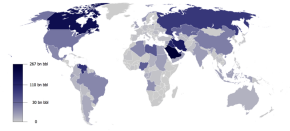 |
| Worldwide Oil Reserves (2009) |
Due to its high
energy density, easy
transportability and
relative abundance, oil
has become the world's most important source of energy since the mid-1950s.
Petroleum is also the raw material for many chemical products,
including pharmaceuticals,
solvents,
fertilizers,
pesticides, and
plastics; the 16% not
used for energy production is converted into these other materials.
Petroleum is found in
porous
rock formations in the
upper
strata of some areas of
the
Earth's
crust. There is also
petroleum in
oil sands (tar sands).
Known
reserves of petroleum
are typically estimated at around 190 km3
(1.2
trillion
(short scale)
barrels) without oil
sands, or 595 km3
(3.74 trillion barrels) with oil sands.
Consumption is currently around 84 million barrels (13.4×10^6 m3)
per day, or 4.9 km3
per year.
Origin
According to generally accepted theory, petroleum is derived from ancient biomass. It is a fossil fuel derived from ancient fossilized organic materials. The theory was initially based on the isolation of molecules from petroleum that closely resemble known biomolecules.
More specifically, crude oil and natural gas are products of heating of ancient organic materials (i.e. kerogen) over geological time. Formation of petroleum occurs from hydrocarbon pyrolysis, in a variety of mostly endothermic reactions at high temperature and/or pressure. Today's oil formed from the preserved remains of prehistoric zooplankton and algae, which had settled to a sea or lake bottom in large quantities under anoxic conditions (the remains of prehistoric terrestrial plants, on the other hand, tended to form coal). Over geological time the organic matter mixed with mud, and was buried under heavy layers of sediment resulting in high levels of heat and pressure (diagenesis). This process caused the organic matter to change, first into a waxy material known as kerogen, which is found in various oil shales around the world, and then with more heat into liquid and gaseous hydrocarbons via a process known as catagenesis.
Geologists often refer to the temperature range in which oil forms as an "oil window"—below the minimum temperature oil remains trapped in the form of kerogen, and above the maximum temperature the oil is converted to natural gas through the process of thermal cracking. Sometimes, oil which is formed at extreme depths may migrate and become trapped at much shallower depths than where it was formed. The Athabasca Oil Sands is one example of this.
Crude Oil Reservoirs
Three conditions must be present for oil reservoirs to form: a source rock that is rich in hydrocarbon material buried deep enough for subterranean heat to cook it into oil; a porous
_svg_small.png) |
| Hydrocarbon Trap (Green=Shale, Red=Oil, Yellow=Sand) (Picture Source) |
The reactions that produce oil and natural gas are often modeled as first order breakdown reactions, where hydrocarbons are broken down to oil and natural gas by a set of parallel reactions, and oil eventually breaks down to natural gas by another set of reactions. The latter set is regularly used in petrochemical plants and oil refineries.
People drill articificial oil wells into oil reservoirs to extract the crude oil and the natural gas. "Natural lift" production methods that rely on the natural reservoir pressure to force the oil to the surface are usually sufficient for a while after reservoirs are first tapped. In some reservoirs, such as in the Middle East, the natural pressure is sufficient over a very long time. The natural pressure in many reservoirs, however, is eventually used up. Then, any more oil to be recovered must be either pumped out using an “artificial lift” created by mechanical pumps powered by internal-combustion engines or electric motors, or by the pressure of large amounts of ordinary water or natural gas injected into the oil reservoir. Over time, these "primary" methods become less effective and "secondary" production methods may be used. A common secondary method is “waterflood” or injection of water into the reservoir to increase pressure and force the oil to the drilled shaft or "wellbore." Eventually "tertiary" or "enhanced" oil recovery methods may be used to increase the oil's flow characteristics by injecting steam, carbon dioxide, or other gases or chemicals into the reservoir. In the United States, primary production methods usually account for less than 40% of the oil produced on a daily basis, secondary methods account for about half, and tertiary recovery the remaining 10%. Extracting oil (or “bitumen”) from oil/tar sand and oil shale deposits requires mining the sand or shale and heating it in a vessel or retort, or using “in-situ” methods of injecting heated liquids into the deposit and then pumping out the oil-saturated liquid.
End of Lesson
Return to the Old Earth Ministries Online Earth History Curriculum homepage.