Because so much
biodiversity was lost, the recovery of life on earth took significantly
longer than after other extinction events. This event has been described as
the "mother of all mass extinctions". The
pattern of extinction is still disputed, as
different studies suggest one to three different
pulses. There are several proposed mechanisms for the extinctions; the
earlier peak was likely due to gradualistic
environmental change, while the latter was probably due to a catastrophic
event. Possible mechanisms for the latter include large or multiple
bolide
impact events, increased
volcanism, or sudden release of
methane hydrates from
the sea floor; gradual changes include sea-level change,
anoxia, increasing
aridity, and a shift in ocean circulation
driven by climate change.
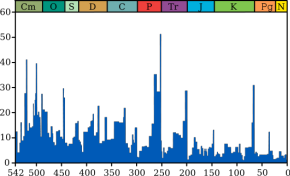
Dating the extinction
Until about
the year 2000 it was thought that rock sequences spanning the
Permian-Triassic boundary were too few and contained too many gaps for
scientists to estimate reliably when the extinction occurred, how long it
took or whether it happened at the same time all over the world.
However, a study of
uranium/lead ratios of
zircons from rock
sequences near Meishan, Changxing, Zhejiang Province, China
date the extinction to
251.4 ±0.03 Ma,
with an ongoing elevated extinction rate occurring for some time thereafter.
A large (-9‰), abrupt global change in the
ratio of
13C
to
12C,
denoted δ13C,
coincides with this extinction, and is sometimes
used to identify the Permian-Triassic boundary in rocks that are unsuitable
for radiometric dating.
It has been
suggested that the Permian-Triassic boundary is associated with a sharp
increase in the abundance of marine and terrestrial
fungi, and that this
was caused by the sharp increase in the amount of dead plants and animals
fed upon by the fungi. For a while this "fungal
spike" was used by some paleontologists to identify the boundary to define
the Permian-Triassic boundary in rocks that are unsuitable for radiometric
dating or lack suitable
index fossils, but even
the proposers of the fungal spike hypothesis pointed out that "fungal
spikes" may have been a repeating phenomenon created by the post-extinction
ecosystem in the earliest Triassic. More
recently the very idea of a fungal spike has been criticized on several
grounds, including that: Reduviasporonites,
the most common supposed "fungal spore", was actually a fossilized
alga; the spike did not
appear worldwide; and in many places it did not
fall on the Permian-Triassic boundary. The algae
which were mis-identified as fungal spores may even represent a transition
to a lake-dominated Triassic world rather than an earliest Triassic zone of
death and decay in some terrestrial fossil beds.
However, newer chemical evidence agrees better with a fungal origin for
Reduviasporonites,
diluting these critiques.
Uncertainty
exists regarding the duration of the overall extinction and about the timing
and duration of various groups' extinctions within the greater process. Some
evidence suggests that the extinction was spread out over a few million
years, with a very sharp peak in the last 1 million years of the Permian.
Statistical analyses of some highly fossiliferous strata in Meishan, South
China suggest that the main extinction was clustered around one peak.
Recent research shows that different groups went extinct at different times;
for example, while difficult to date absolutely,
ostracode and
brachiopod extinctions
were separated by between 0.72 and 1.22 million years.
In a well preserved sequence in east Greenland, the decline of animals is
concentrated in a period 10 to 60 thousand years long, with plants taking
several hundred thousand further years to show the full impact of the event.
An older theory, still supported in some recent papers,
is that there were two major extinction pulses 5 million years apart,
separated by a period of extinctions well above the background level; and
that the final extinction killed off "only" about 80% of marine species
alive at that time while the other losses occurred during the first pulse or
the interval between pulses. According to this theory the first of these
extinction pulses occurred at the end of the
Guadalupian
epoch of the Permian. For example,
all but one of the surviving
dinocephalian genera
died out at the end of the Guadalupian, as did
the Verbeekinidae, a family of large-size
fusuline
foraminifera. The
impact of the end-Guadalupian extinction on marine organisms appears to have
varied between locations and between taxonomic groups - brachiopods and
corals had severe losses.
The
event had a profound effect on the terrestrial ecosystem, which is still
being felt today, a quarter of a billion years later.
In the late Permian, there were many sorts of
Marine invertebrates
suffered the greatest losses during the P–Tr extinction. In the
intensively-sampled south China sections at the P-Tr boundary, for instance,
280 out of 329 marine invertebrate genera disappear within the final 2
sedimentary zones containing
conodonts from the
Permian.
Statistical
analysis of marine losses at the end of the Permian suggests that the
decrease in diversity was caused by a sharp increase in extinctions instead
of a decrease in speciation. The
extinction primarily affected organisms with calcium carbonate skeletons,
especially those reliant on ambient CO2
levels to produce their skeletons.
Among
benthic organisms, the extinction event multiplied background extinction
rates, and therefore caused most damage to taxa that had a high background
extinction rate (by implication, taxa with a high turnover).
The extinction rate of marine organisms was catastrophic.
Marine
invertebrate groups which survived include: articulate
brachiopods (those with
a hinge), which have suffered a slow decline in numbers since the P–Tr
extinction; the
Ceratitida order of
ammonites; and
crinoids ("sea
lilies"), which very nearly became extinct but later became abundant and
diverse.
The groups
with the highest survival rates generally had active control of circulation,
elaborate gas exchange mechanisms, and light calcification; more heavily
calcified organisms with simpler breathing apparatus were the worst hit.
In the case of the brachiopods at least, surviving taxa were generally
small, rare members of a diverse community.
The
ammonoids, which had
been in a long-term decline for the 30 million years since the Roadian
(middle Permian), suffered a selective end-Guadalupian extinction pulse.
This extinction greatly reduced disparity, and suggests that environmental
factors were responsible for this extinction. Diversity and disparity fell
further until the P-Tr boundary; the extinction here was non-selective,
consistent with a catastrophic initiator. During the Triassic, diversity
rose rapidly, but disparity remained low.
The range of
morphospace occupied by
the ammonoids became more restricted as the Permian progressed. Just a few
million years into the Triassic, the original morphospace range was once
again occupied, but shared differently between clades.
Terrestrial
invertebrates
The Permian
had great diversity in insect and other invertebrate species, including the
largest insects ever to have existed. The end-Permian is the only known mass
extinction of insects, with eight or nine insect
orders becoming extinct and ten more greatly reduced in diversity.
Palaeodictyopteroids
(insects with piercing and sucking mouthparts) began to decline during the
mid-Permian; these extinctions have been linked to a change in flora. The
greatest decline, however, occurred in the Late Permian and were probably
not directly caused by weather-related floral transitions.
Most fossil
insect groups which are found after the Permian–Triassic boundary differ
significantly from those which lived prior to the P–Tr extinction. With the
exception of the
Glosselytrodea,
Miomoptera, and
Protorthoptera,
Paleozoic insect groups have not been discovered in deposits dating to after
the P–Tr boundary. The
caloneurodeans,
monurans,
paleodictyopteroids,
protelytropterans, and
protodonates became
extinct by the end of the Permian. In well-documented Late Triassic
deposits, fossils overwhelmingly consist of modern fossil insect groups.
This is a long lesson. This point is a good
division point to turn it into two lessons if you desire.
Terrestrial plants
Plant
ecosystem response
The
geological record of terrestrial plants is sparse, and based mostly on
pollen and
spore studies.
Interestingly, plants are relatively immune to mass extinction, with the
impact of all the major mass extinctions "negligible" at a family level.
Even the reduction observed in species diversity (of 50%) may be mostly due
to
taphonomic processes.
However, a massive rearrangement of ecosystems does occur, with plant
abundances and distributions changing profoundly.
At the P–Tr
boundary, the dominant floral groups changed, with many groups of land
plants entering abrupt decline, such as
Cordaites (gymnosperms)
and
Glossopteris (seed
ferns). Dominant
gymnosperm genera were
replaced post-boundary by
lycophytes - extant
lycophytes are recolonizers of disturbed areas.
Palynological or pollen studies from East Greenland of
sedimentary rock strata laid down during the extinction period indicate
dense gymnosperm woodlands before the
event. At the same time that marine invertebrate macrofauna are in decline
these large woodlands die out and are followed by a rise in diversity of
smaller
herbaceous plants
including
Lycopodiophyta, both
Selaginellales and
Isoetales. Later on
other groups of gymnosperms again become dominant but again suffer major die
offs; these cyclical flora shifts occur a few times over the course of the
extinction period and afterwards. These fluctuations of the dominant flora
between woody and herbaceous taxa indicate chronic environmental stress
resulting in a loss of most large woodland plant species. The successions
and extinctions of plant communities do not coincide with the shift in δ13C
values, but occurs many years after. The
recovery of gymnosperm forests would take 4-5 million years.
The
Coal Gap
No
coal deposits are known
from the Early Triassic, and those in the Middle Triassic are thin and
low-grade. This "coal gap" has been explained in
many ways. It has been suggested that new, more aggressive fungi, insects
and vertebrates evolved, and killed vast amounts of trees. However these
decomposers themselves suffered heavy losses of species during the
extinction, and are not considered a likely cause of the coal gap.
It could simply be that all coal forming plants were rendered extinct by the
P/Tr extinction, and that it took 10 million years for a new suite of plants
to adapt to the moist, acid conditions of peat bogs.
On the other hand abiotic factors (not caused by organisms), such as
decreased rainfall or increased input of clastic sediments, may also be to
blame. Finally, it is also true that there are
very few sediments of any type known from the Early Triassic, and the lack
of coal may simply reflect this scarcity. This opens the possibility that
coal-producing
ecosystems may have
responded to the changed conditions by relocating, perhaps to areas where we
have no sedimentary record for the Early Triassic.
For example in eastern Australia a cold climate had been the norm for a long
period of time, with a peat
mire ecosystem
specialising to these conditions. Approximately 95% of these peat-producing
plants went locally
extinct at the P-Tr boundary;
Interestingly, coal deposits in Australia and Antarctica disappear
significantly before
the P-Tr boundary.
Terrestrial
vertebrates
Even the
groups that survived suffered extremely heavy losses of species, and some
terrestrial vertebrate groups very nearly became extinct at the end-Permian.
Some of the surviving groups did not persist for long past this period,
while others that barely survived went on to produce diverse and
long-lasting lineages. There is enough evidence to indicate that over
two-thirds of terrestrial
amphibian,
sauropsid ("reptile")
and
therapsid ("mammal-like
reptile")
families became
extinct. Large herbivores suffered the heaviest losses. All Permian
anapsid reptiles died
out except the
procolophonids (testudines
have anapsid skulls but are most often thought to have evolved later, from
diapsid ancestors).
Pelycosaurs died out
before the end of the Permian. Too few Permian
diapsid fossils have
been found to support any conclusion about the effect of the Permian
extinction on diapsids (the "reptile" group from which lizards, snakes,
crocodilians,
dinosaurs, and birds
evolved).
Possible explanations of these patterns
The most
vulnerable marine organisms were those which produced calcareous hard parts
(i.e. from
calcium carbonate) and
had low
metabolic rates and
weak respiratory systems - notably calcareous sponges, rugose and tabulate
corals, calciate brachiopods, bryozoans, and echinoderms; about 81% of such
genera became extinct.
Close relatives which did not produce calcareous hard parts suffered only
minor losses, for example
sea anemones, from
which modern corals later evolved. Animals which had high metabolic rates,
well-developed respiratory systems and non-calcareous hard parts had
negligible losses - except for
conodonts, in which 33%
of genera died out.
This pattern
is consistent with what is known about the effects of
hypoxia, a shortage but
not a total absence of
oxygen. However,
hypoxia cannot have been the only killing mechanism for marine organisms.
Nearly all of the
continental shelf
waters would have had to become severely hypoxic to account for the
magnitude of the extinction, but such a catastrophe would make it difficult
to explain the very selective pattern of the extinction.
Models of the Late
Permian and Early Triassic atmospheres show a significant but protracted
decline in atmospheric oxygen levels, with no acceleration near the P-Tr
boundary. Minimum atmospheric oxygen levels in the Early Triassic are never
less than present day levels - the decline in oxygen levels does not match
the temporal pattern of the extinction.
The observed pattern of marine
extinctions is also consistent with
hypercapnia (excessive
levels of
carbon dioxide). Carbon
dioxide (CO2)
is actively toxic at above-normal concentrations, as it reduces the ability
of
respiratory pigments to
oxygenate tissues, and makes body fluids more
acidic, thereby
hampering the production of
carbonate hard parts
like shells. At high concentrations, carbon dioxide causes
narcosis
(intoxication). In addition to these direct effects, CO2
reduces the concentration of carbonates in water by "crowding them out",
which further increases the difficulty of producing
carbonate hard parts.
Marine organisms are more sensitive
to changes in CO2
levels than are terrestrial organisms for a variety of reasons. CO2
is 28 times more soluble in water than is oxygen. Marine animals normally
function with lower concentrations of CO2
in their bodies than land animals, as the removal of CO2
in air-breathing animals is impeded by the need for the gas to pass through
the respiratory systems membranes (lungs,
tracheae, and the
like). In marine organisms, relatively modest but sustained increases in CO2
concentrations hamper the synthesis of
proteins, reduce
fertilization rates, and produce deformities in calcareous hard parts.
It is difficult to analyze extinction
and survival rates of land organisms in detail, because there are few
terrestrial fossil beds that span across the Permian-Triassic boundary.
Triassic insects are very different from those of the Permian, but there is
a gap in the insect fossil record spanning approximately 15M years from the
late Permian to early Triassic. The best known record of vertebrate changes
across the Permian-Triassic boundary occurs in the
Karoo Supergroup of
South Africa, but statistical analyses have so far not produced clear
conclusions.
Biotic
recovery
Earlier
analyses indicated that life on Earth recovered quickly after the Permian
extinctions, but this was mostly in the form of
disaster taxa, such as
the hardy
Lystrosaurus. The most
recent research indicates that the specialized animals that formed complex
ecosystems, with high biodiversity, complex food webs and a variety of
niches, took much longer to recover. It is thought that this long recovery
was due to the successive waves of extinction which inhibited recovery, as
well as to prolonged environmental stress to organisms which continued into
the Early Triassic. Recent research indicates that recovery did not begin
until the start of the mid-Triassic, 4 million to 6 million years after the
extinction; and some writers estimate that the
recovery was not complete until 30M years after the P-Tr extinction, i.e. in
the
late Triassic.
During the
early Triassic (4-6M years after the P-Tr extinction), the plant biomass was
insufficient to form coal deposits, which
implies a limited food mass for herbivores.
River patterns in the
Karoo changed from
meandering to
braided, indicating
that vegetation there was very sparse for a long time.
Each major
segment of the early Triassic ecosystem — plant and animal, marine and
terrestrial — was dominated by a small number of
genera, which appeared
virtually worldwide, for example: the herbivorous
therapsid
Lystrosaurus (which
accounted for about 90% of early Triassic land vertebrates) and the
bivalves Claraia,
Eumorphotis,
Unionites and
Promylina. A healthy
ecosystem has a much
larger number of genera, each living in a few preferred types of habitat.
Disaster
taxa (opportunist organisms) took advantage of the devastated ecosystem and
enjoyed a temporary population boom and increase in their territory. For
example:
Lingula (a
brachiopod);
stromatolites, which
had been confined to marginal environments since the
Ordovician;
Pleuromeia (a small,
weedy plant);
Dicroidium (a
seed fern).
Changes
in marine ecosystems
Prior to the extinction,
approximately 67% of marine animals were sessile and attached to the sea
floor, but during the Mesozoic only about half of the marine animals were
sessile while the rest were free living. Analysis of marine fossils from the
period indicated a decrease in the abundance of
sessile epifaunal
suspension feeders,
such as
brachiopods and
sea lilies, and an
increase in more complex mobile species such as
snails,
urchins and
crabs.
Before the Permian mass extinction
event, both complex and simple marine ecosystems were equally common; after
the recovery from the mass extinction, the complex communities outnumbered
the simple communities by nearly three to one,
and the increase in predation pressure led to the
Mesozoic Marine Revolution.
Bivalves were fairly
rare before the P–Tr extinction but became numerous and diverse in the
Triassic and one group, the
rudist clams, became
the
Mesozoic's main
reef-builders. Some researchers think much of this change happened in the 5
million years between the two major extinction pulses.
Crinoids ("sea lilies")
suffered a selective extinction, resulting in a decrease in the variety of
forms in which they grew. Their ensuing
adaptive radiation was
brisk, and resulted in forms possessing flexible arms becoming widespread;
motility, predominantly a response to predation pressure, also became far
more prevalent.
Land
vertebrates
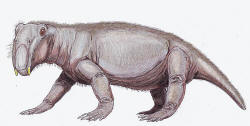
Lystrosaurus, a
pig-sized herbivorous
dicynodont
therapsid, constituted
as much as 90% of some earliest Triassic land vertebrate faunas.
Smaller carnivorous
cynodont
therapsids also
survived, including the ancestors of
mammals. In the
Karoo region of
southern
Africa the
therocephalians
Tetracynodon,
Moschorhinus and
Ictidosuchoides
survived but do not appear to have been abundant in the Triassic.
Archosaurs (which
included the ancestors of dinosaurs and
crocodilians) were
initially rarer than therapsids, but they began to displace therapsids in
the mid-Triassic. In the mid to late Triassic
the
dinosaurs evolved from
one group of archosaurs, and went on to dominate terrestrial ecosystems for
the rest of the
Mesozoic. This
"Triassic Takeover" may have contributed to the
evolution of mammals by
forcing the surviving therapsids and their
mammaliform successors
to live as small, mainly nocturnal
insectivores; nocturnal
life probably forced at least the mammaliforms to develop fur and higher
metabolic rates.
Some
temnospondyl
amphibians made a
relatively quick recovery, in spite of nearly becoming extinct.
Mastodonsaurus and
trematosaurians were
the main aquatic and semi-aquatic predators during most of the
Triassic, some preying
on
tetrapods and others on
fish.
Land vertebrates took an unusually
long time to recover from the P-Tr extinction; one writer estimates that the
recovery was not complete until 30 million years after the extinction, i.e.
not until the Late Triassic, in which dinosaurs,
pterosaurs, crocodiles,
archosaurs, amphibians and mammaliforms were abundant and diverse.
End of Reading

Return to the
Old Earth Ministries Online Earth
History Curriculum homepage.

Source Pages: Wikipedia -
Permian,
Permian Extinction